Choose the Answer That Best Describes Hco3-.
Choose the statment that is false or incorrect. 81 Choose the answer that best describes HCO3-.

Anatomy And Physiology 5th Edition Marieb Test Bank By Xteamemail34 Issuu
3 upper O subscript 2 g right arrow 2 upper O subscript 3 g.

. A glucose molecules joined to make glycogen. Select which reactions will usually be irreversible regarding chemical equilibrium in human bodies. A 7 _____ is fat soluble produced in the skin on exposure to UV radiation and necessary for normal bone growth and function.
Has the same acid strength. A a bicarbonate io n. Choose the answer that best describes HCO3-.
6Choose the answer that best describes HCO3-. E cholic and chenodeoxycholic acid. HCO 3 -CO3 2- H.
A a bicarbonate ion B a proton donor C common in the liver D a weak acid. I will give Brainliest When a hydrogen carbonate ion reacts with water water acts as a Brønsted-Lowry base. A a bicarbonate ion Select which reactions will usually be irreversible regarding chemical equilibrium in living systems.
A a bicarbonate ion B common in the liver C a weak acid D a proton donor. The conjugate base of HCO3- bicarbonate ion is CO32- carbonate ion The conjugate acid of HCO3- bicarbonate ion is H2CO3 carbonic acid What phrase best describes a thesis. Answer not in Detail.
Delta H 1 equals negative 483. H2O CO2 H2CO3 HCO3 H During hyperventilation the CO2 levels in the circulation decrease so the dissociation of carbonic acid H2CO3 into bicarbonate and hydrogen ions reduce. Choose the answer that best describes HCO3-.
1 Get Other questions on the subject. Choose the answer that best describes HCO3-a biocarbonate ion. Choose from the listed substances components that participate in the digestion of fats.
A a bicarbonate ion B common in the liver C a weak acid D a proton donor Answer. The conjugate base of any compound is the compound formed after the removal of H from them respectively. Thus the conjugate base of acid H 2 O is OH.
Choose the answer that best describes HCO3- a bicarbonate 25 Select which reactions will usually be irreversible regarding chemical equilibrium in living system. Glucose to CO2 and H2O 26 What happens in redox reactions. 6 kilojoules divided by 2 equals 142.
HCO3aq H2Ol H3Oaq CO32aq B. H2CO3aq H2Ol OHaq H3CO3aq. Melissa probably more closely resembles.
AnswerD 81Choose the answer that best describes HCO3-. Which phase best. A a proton donor B a weak acid C a bicarbonate ion D common in the liver Answer.
Your Feedback will Help us Serve you better. A a bicarbonate ion B common in the liver C a weak acid D a proton donor Answer. What is a dipole.
Solution for Choose the phrase that best describes the relative acid strength of these acids. Choose An Option That Best Describes Your Problem. A a weak acid B a bicarbonate ion C common in the liver D a proton donor.
Choose the answer that best describes HCO3-. All of the choices rae correct Reason. AADP Pi to make ATP Bglucose to CO2 and H2O Cglucose molecules joined to make glycogen DH2O CO2 to make H2CO3.
A B C D 82 Select which. 13 Select which reactions will usually be irreversible regarding chemical equilibrium in human bodies. Choose the reaction that describes this.
D taurocholic and glycocholic acids. Which term best describes hco3-polyatomic anion polyatomic cation monatomic anion monatomic cation need asap. Both decomposition and electron exchange occur 27.
Choose the answer that best describes HCO3-. Melissa is interested in her family tree and how her family has changed over its many generations. H 2 O H OH.
Delta H 2 equals 284. A a weak acid B common in the liver C a bicarbonate ion D a proton donor. Is more acidic than HSe HS.
Chemistry 22062019 0930 kkqueen01. A a bicarbonate ion. A 12 Choose the answer that best describes HCO3-.
Aa proton donor Ba bicarbonate ion Ca weak acid Dcommon in the liver AnswerB 82Select which reactions will usually be irreversible regarding chemical equilibrium in human bodies. Leave a Comment Cancel reply. A glucose to CO2 and H2O B ADP Pi to make ATP CH2O CO2 to make H2CO3.
8 kilojoules per mole. Your Mobile number and Email id will not be published. A Vitamin K B Cortisol.
6 kilojoules divided by 2 equals negative 241. Answer not in Detail. 3 kilojoules per mole.
A key feature of the bodys metabolism is the almost exclusive use of exergonic reactions by the body. Thus the conjugate base of acid HCO3- is CO3 2-.

Solved Given Your Knowledge Of The Equation Below Which Chegg Com

Rc 212 Midterm Exam Study Guide

Electrochemistry Multiple Choice

Practice Exam In Word 97 Format Right Click To

Solved 7 Ph 7 52 Pco Hco3 28 8 Ph 7 30 Pco Chegg Com

Mastering A P Chapter 1 Chemistry Flashcards Quizlet

Solved Which Statement Best Describes A Weak Acid A High Chegg Com

Choose The Answer That Best Describes Hco3 A A Proton Donor B A Weak Acid C A Course Hero

Choose The Answer That Best Describes Hco3 A A Proton Donor B A Weak Acid C A Course Hero

Choose The Answer That Best Describes Hco3 A A Proton Donor B A Weak Acid C A Course Hero

Choose The Answer That Best Describes Hco3 A A Proton Donor B A Weak Acid C A Course Hero
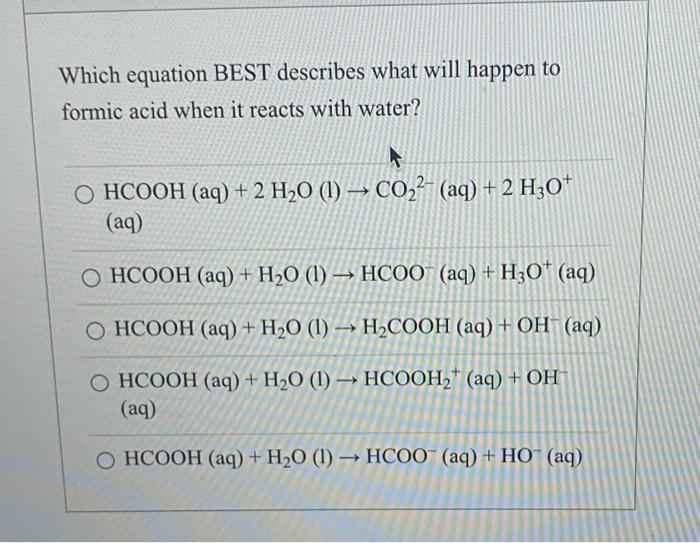
Solved Which Equation Best Describes What Will Happen To Chegg Com

Solved Responses To Auditory Cues Are Than Responses To Chegg Com

Choose The Answer That Best Describes Hco3 A A Proton Donor B A Weak Acid C A Course Hero
Solved Which Of The Following Best Describes Eicosanoids Chegg Com

Choose The Answer That Best Describes Hco3 A A Proton Donor B A Weak Acid C A Course Hero


Comments
Post a Comment